An introduction to SEDIMENTARY ROCKS
Sedimentary rocks form from sediment.
"Sediment" is a general term that indicates loose grains (such as the sand at the beach) and chemical precipitates (such as crystals of salt).
Sedimentary rocks can be subdivided in three categories: clastic, chemical, and biochemical sedimentary rocks.
A. CLASTIC (or DETRITAL) SEDIMENTARY ROCKS
Clastic sedimentary rocks form when individual grains (the sediment), made by fragments of rocks, minerals, or shells, are cemented together (the clastic sediemntary rock). These are the only type of rock that is NOT made simply of crystals, but rather fragments of other materials, broken and then reassembled together.
Loose grains (also called clasts) are produced by the action of chemical and physical weathering; these grains are classified according to their size in gravel, sand, silt, and clay.
The grains are eroded (removed from their original location), transported (mostly by water in streams, but also by winds and by glaciers), and finally deposited.
This cycle of erosion-transportation-deposition can be repeated several times, thus increasing:
- the compositional maturity of the sediment (most of the grains will slowly be reduced to a quartz and clay minerals composition).
- the sorting of the sediment (most of the grains will be separated one form the other according tho their size; or, most stream deposits will be made by sediment with approximately the same size)
- the rounding of the sediment (most of the grains will be smooth and not angular). Please note that rounding should not be confused with sphericity (see your book for details).
The clastic sediment grains will eventually be covered by younger sediment and become a virtually permanent deposit. This implies:
- burial of the sediment
- compaction of the sediment (the pore spaces between the grains will be reduced by the overlying load),
- cementation of the sediment cemented (water circulating through the pores will release a chemical compound, usually quartz, calcite, or hematite) into what has finally become a clastic sedimentary rock.
Burial, compaction, and cementation are the steps that lead to the process of lithification, literally "the making of a rock".
The most common clastic sedimentary rocks are breccia and conglomerate (formed from gravel), sandstone (from sand), siltstone (from silt), and shale (from clay)
B. CHEMICAL SEDIMENTARY ROCKS
Chemical sedimentary rocks are crystalline, that is made of crystals interlocked with each other. Conceptually, that is the same texture we see in igneous and metamorphic rocks, but NOT in clastic sedimentary rocks.
Chemical sedimentary rocks form because of the precipitation of crystals from aqueous (water) solutions and from chemical residues.
When crystals grow out of a solution (for instance, halite, or table salt, out of water) they can only use the space they have available, often interfering with each other's growth pattern.
Some crystals form because of a simple variation in the concentration of ions in presence of water (for instance, calcite and aragonite),
while others require evaporation of water (for instance, halite, or gypsum), and others still form as chemical residues (for instance, some iron oxides).
The most common chemical sedimentary rocks are
- carbonates, whether inorganic limestones (such as travertine, tufa, oolite) or dolostones
- cherts (composed of microcrystalline quartz)
- evaporites (such as halite, gypsum, and anhydrite)
- iron oxides (such as hematite and limonite)
C. BIOCHEMICAL SEDIMENTARY ROCKS
Biochemical sedimentary rocks are formed mostly by the remains (whole or broken) of organisms. These remains can be of different kind and include, but are not limited to, for instance, shells, reefs and corals, structures like echinoids' stems, plant fragments, carbon, and many more.
Since most organisms use calcite or aragonite (calcium carbonate) to build their shells or part of their mineralized structure, it is no surprise that most of the biochemical sedimentary rocks we find can also be classified as limestones
(for instance, coquina, fossiliferous limestones, calcarenite, chalk, micrite, etc.).
Other organisms, like diatoms, use silica to make their shells, and a rock forming out o ftheir remians will be known as a siliceous diatomite.
Peat, lignite, and coal form from progressive concentration of organic matter (carbon) in plant fragments that have not been oxidized.
Common Clastic, or Detrital, Sedimentary Rocks Textures
Most images contain a coin, or a lens cap, or a cm-based ruler for scale and clarity
1 
CLASTIC TEXTURE
Visible grains, coarser than sand; grains are angular
(grain size > 2 mm)
Breccia |
2 
CLASTIC TEXTURE
Visible grains, coarser than sand; grains are rounded
(grain size > 2 mm)
Conglomerate |
3 
CLASTIC TEXTURE
Visible grains, coarse sand-size
(grain size between 1/16 and 2 mm)
Arkose Sandstone |
4 
CLASTIC TEXTURE
Visible grains, sand-size;
the rock feels gritty to the touch
(grain size between 1/16 and 2 mm)
Quartz Sandstone |
5 
CLASTIC TEXTURE
The grains barely visible;
the rock feels mostly smooth to the touch
(grain size between 1/256 and 1/16 mm)
Siltstone |
6 
CLASTIC TEXTURE
The grains are not visible;
the rock feels smooth to the touch
(grain size smaller than 1/256 mm)
Mudstone (clay and silt) |
7 
CLASTIC TEXTURE
The grains are not visible,
but fissility is very evident
Shale |
|
|
All pictures © Alessandro Grippo
Pictures 1 through 5: Samples from the Santa Monica College collection. Santa Monica, California
Picture 6: Mudstone (with mud cracks) draping ripples formed on sand dunes. Colorado Plateau, Emery County, Utah
Picture 7: Shale outcrop from Mosaic Canyon, Death Valley. Inyo County, California
Common Chemical Sedimentary Rocks Textures
most images contain a cm-scale for clarity
8 
CRYSTALLINE TEXTURE
Microcrystalline calcite (CaCO3)
(fossil leaf encrusted by calcite)
Travertine (Limestone), encrusting |
9 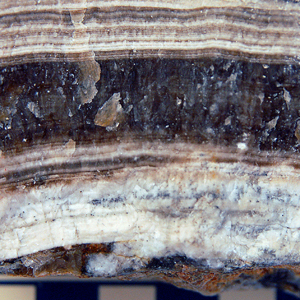
CRYSTALLINE TEXTURE
Layered microcrystalline calcite (CaCO3)
(dark colors probably caused by organic matter in calcite)
Travertine (Limestone), with growth bands |
10 
CRYSTALLINE TEXTURE
Individual calcite ooids have been cemented
(dark color probably caused by organic matter)
Oolite (Limestone), fossil |
11 
CRYSTALLINE TEXTURE
Individual calcite ooids have been cemented
(cementation very faint, highly porous limestone)
Oolite (Limestone), recent |
12 
CRYSTALLINE TEXTURE
Microcrystalline quartz (SiO2)
(likely from dissolution and reprecipitation of silica)
Chert |
13 
CRYSTALLINE TEXTURE
Microcrystalline iron oxide (Fe2O3)
(from oxidation of Fe-rich minerals)
Hematite |
14 
CRYSTALLINE TEXTURE
Microcrystalline halite (NaCl)
(crystal size less than 1 cm)
Halite, microcrystalline |
15 
CRYSTALLINE TEXTURE
Macrocrystalline cubic halite (NaCl)
(crystal size about 1cm)
Halite, sub-cm size crystals |
16 
CRYSTALLINE TEXTURE
Microcrystalline halite (NaCl)
(red color caused by impurities in the salt)
Halite, microcrystalline |
17 
CRYSTALLINE TEXTURE
Macrocrystalline, geminate gypsum (CaSO4·2H2O)
(lens cover for scale)
Gypsum, "swallow tail" crystals |
18 
CRYSTALLINE TEXTURE
Gypsum crystal and microcrystalline gypsum
(CaSO4·2H2O)
Gypsum, crystalline (left) and amorphous (right) |
All pictures © Alessandro Grippo
Picture 8: Travertine. Walls of the J. Paul Getty Center, Los Angeles. (Travertine imported from Rome, Italy)
Pictures 9 through 13: Samples from the Santa Monica College collection, Santa Monica, California
Picture 14: Halite (Salt) from the Bonneville Salt Flats, Wendover, Tooele County, Utah
Picture 15: Halite (Salt) from Searles Lake, Trona, San Bernardino County, California
Picture 16: Sample from the Santa Monica College collection, Santa Monica, California
Picture 17: Gypsum. Basal walls of medieval tower, downtown Bologna, Italy
Picture 18: Samples from the Santa Monica College collection, Santa Monica, California
Common Biochemical Sedimentary Rocks Textures
most images contain a coin or a cm-based ruler for scale and clarity
19 
BIOCLASTIC (CRYSTALLINE) TEXTURE
Microscopic calcitic shells of protists, weakly cemented
(CaCO3)
Chalk (Limestone) |
20 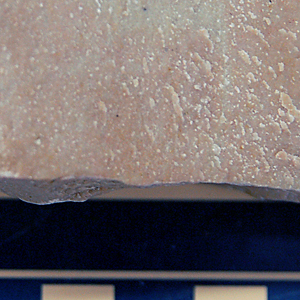
CRYSTALLINE TEXTURE
Microcrystalline calcite (CaCO3)
(from recrystallization of chalk)
Micrite (Limestone) |
21 
BIOCLASTIC (CRYSTALLINE) TEXTURE
Mix of clastic materials and calcitic shells
(evident gastropods fossils, shells made of CaCO3)
Fossiliferous Limestone |
22 
CRYSTALLINE TEXTURE
Coral reef fragment (CaCO3)
(calcite secreted by reef-builders polyps)
fossiliferous limestone |
23 
BIOCLASTIC TEXTURE
Fragments of calcitic shells (CaCO3)
(shells have been broken and fragments concentrated)
Coquina (limestone) |
24 
BIOCLASTIC (CRYSTALLINE) TEXTURE
Microcrystalline carbon (C)
(carbon concentrated from plants remains)
Coal |
25 
BIOCLASTIC TEXTURE
Whole and fragmented shells of marine organisms
made of calcite (CaCO3)
(see the two images on the side for an enlarged version)
Coquina (Limestone)
|
26 
BIOCLASTIC TEXTURE
Whole and fragmented shells of marine organisms
made of calcite (CaCO3)
(enlargement of the previous picture; see coin for scale) Coquina (Limestone) |
27 
BIOCLASTIC TEXTURE
Whole and fragmented shells of marine organisms
made of calcite (CaCO3)
(enlargement of the previous picture; see coin for scale) Coquina (Limestone) |
All pictures © Alessandro Grippo
Pictures 19 through 24: Samples from the Santa Monica College collection, Santa Monica, California
Pictures 24 through 26: Coquina. Fortification walls, St. Augustine, St. Johns County, Florida
Go back to the INTRODUCTION TO ROCKS page
Go to the IGNEOUS ROCKS page
Go to the METAMORPHIC ROCKS page

Go back to the MAIN PAGE page
Go back to the IMAGES page
© Alessandro Grippo, since 1994
last updated: September 26, 2017LEGAL NOTICE
| |




























